Svetlana N. Radyuk
Research Associate Professor
Biological Sciences
| Office Location |
DLSB 340 |
| Phone |
214-768-2892 |
Education
Ph.D. Research Institute for Applied MicrobiologyTel: 214-768-2065
Email: snradyuk@smu.edu
Education
|
1979: |
MS Chemistry, M.V.Lomonosov Moscow State University, Russia |
|
1990: |
PhD Biology Research Institute for Applied Microbiology, Russia |
Teaching
Immunobiology (BIOL 4319)
Molecular Genetics Laboratory (BIOL 3222)
Concepts in Molecular Genetic Investigations (BIOL 6222)
Concepts in Biological Sciences (BIOL 6314)
Research in Biology (BIOL 6370)
Graduate Research (Thesis research) (BIOL 6270)
Dissertation (Research) (BIOL 8398)
Research Interests
Immunity and aging
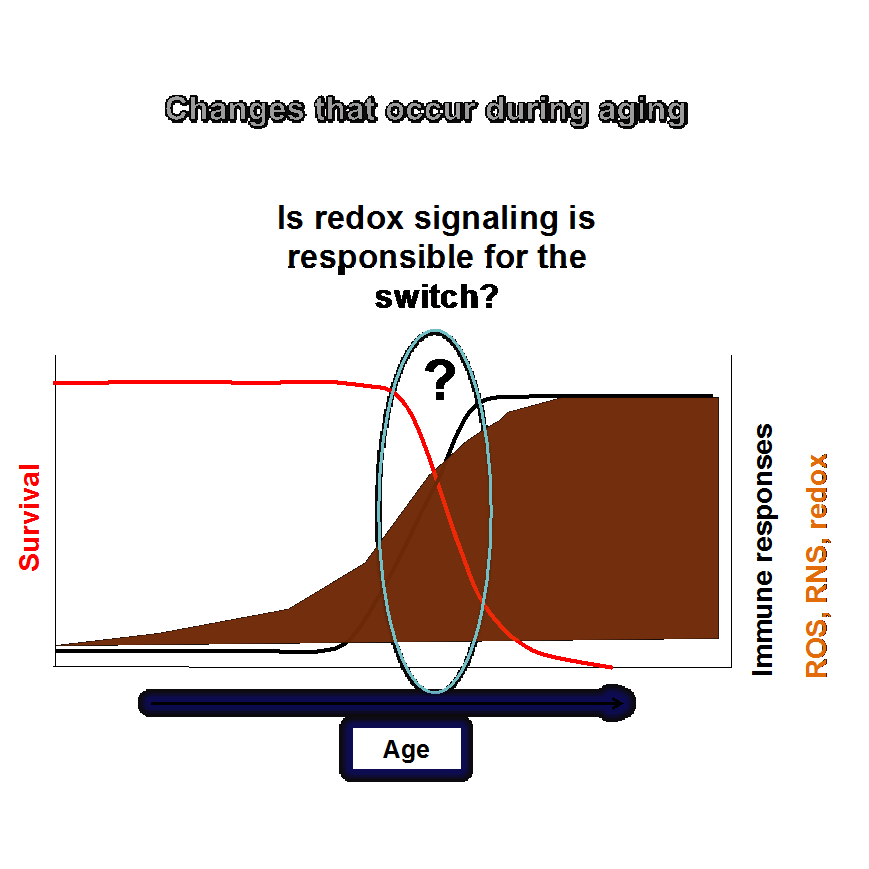
The focus of our lab current research is to understand the role of redox regulation of the innate immunity and aging. Aging is characterized by the malfunction of the immune system. Deregulation of the immune response during aging can result in cancer and other age-related diseases. The study in our group is aimed at elucidating the mechanisms that underlie such deregulation in order to develop proper interventions.
The most dramatic changes associated with aging involve immunity:
• Decreased ability to mount an immune response
• Increased susceptibility to infectious diseases and cancer
• Reduced responsiveness to vaccination
• Impaired function and clonal expansion of T and B cells
• Lower levels of Toll-like Receptors
• Enhanced innate immunity in older organisms give rise to an excessive immune response and inflammation
• Extended longevity correlates with optimal functioning of innate immunity, attenuation of pro-inflammatory responses and decrease in pathogen burden
• Enhanced pathogen resistance is a feature of strains with a long-lived background
The response and resistance of macro-organisms to microbial challenges comes at a cost:
• While bolstered immunity offers protection against invaders, it may also elicit damage to host cells and tissues, which ultimately could lead to functional deficits.
• Excessive production of immunity effectors can also place substantial energy demands on the cellular machinery, which may result in trade-offs that negatively impact the maintenance of cellular homeostasis.

Redox regulation of the immune responses:
Reactive oxygen and nitrogen species (ROS and RNS), produced during metabolic reactions, inflammation, and phagocytosis, not only cause tissue damage, but also act as messengers in signaling pathways, including modulation of the immune responses.
One of the major regulators of cellular redox are enzymes named thiol-dependent peroxidases, or peroxiredoxins. They are:
• widespread in all three kingdoms
• highly conserved in all organisms starting from bacteria and extending into humans
• involved in a number of redox-related cellular functions: differentiation, proliferation, apoptosis, signaling processes, immune response.
The aim is to determine by how to maintain the “correct” levels of ROS/RNS in order to minimize the tissue damage and at the same time not to compromise the immune response. Our research group is testing a hypothesis that peroxiredoxins may control the ‘switch’ in the redox state and thus regulate the immune response and aging. To address these questions, we are using a wide range of molecular biology and genetics techniques.
Practical terms: examine the differences in redox signaling that modulate immunity and aging in both young and old organisms; develop interventions, which take into account the differential effects on young versus old individuals.
Current Support
R01 AG032342 4/1/2011 – 3/31/2016 (Principal Investigator)
“Peroxiredoxins, immune signaling and aging”
Funded by NIH/NIA
The purpose of this study is to determine the role of peroxiredoxins in regulating the relationship between immunity and aging and to identify signaling pathways that define this relationship
R01 AG045830 8/1/2013 – 5/31/2018 (CoPI, PI – Dr. Jadwiga Giebultowicz, Oregon State University)
“Circadian clocks and aging”
Funded by NIH/NIA
The purpose of the study is to determine functional links between redox, strong peripheral circadian clocks and the rate of aging
Selected Publications
Klichko V., Sohal B.H., Radyuk S.N., Orr W.C., Sohal R.S. (2014) Decrease in cytochrome c oxidase reserve capacity diminishes robustness of Drosophila melanogaster and shortens lifespan. Biochem J. 2014 Apr 1;459(1):127-35.
Orr, W.C., Radyuk S.N., Sohal R.S. (2013) Involvement of Redox State in the Aging of Drosophila melanogaster. Antioxid Redox Signal. 2013 Sep 10;19(8):788-803.
Radyuk S.N., Klichko V.I., Michalak K., Orr W.C. (2013) The effect of peroxiredoxin 4 on fly physiology is a complex interplay of antioxidant and signaling functions. FASEB J. 2013 Apr;27(4):1426-38.
Beaver L.M., Klichko V.I., Chow E.S., Kotwica-Rolinska J., Williamson M., Orr W.C., Radyuk S.N., Giebultowicz J.M. (2012) Circadian regulation of glutathione levels and biosynthesis in Drosophila melanogaster. PLoS One. 2012;7(11):e50454. doi: 10.1371/journal.pone.0050454. Epub 2012 Nov 30.
Radyuk, S. N., Gambini, J., Borras, C., Serna, E., Klichko, V. I., Viña, J., Orr, W. C. (2012) Age-dependent changes in the transcription profile of long-lived Drosophila over-expressing glutamate cysteine ligase Mech. Ageing Devel. 2012 Jun;133(6):401-13.
Radyuk SN, Rebrin I, Klichko VI, Sohal BH, Michalak K, Benes J, Sohal RS, Orr WC. (2010) Mitochondrial peroxiredoxins are critical for the maintenance of redox state and the survival of adult Drosophila. Free Radic Biol Med. 49: 1892-1902.
Radyuk, S. N., Rebrin, I., Luchak, J. M., Michalak, K., Klichko, V. I., Sohal, R. S., Orr, W. C. (2008). The catalytic subunit of Drosophila glutamate-cysteine ligase is a nucleo-cytoplasmic shuttling protein. J. Biol. Chem. 2009 Jan 23;284(4):2266-74.
Radyuk SN, Rebrin I, Klichko VI, Sohal BH, Michalak K, Benes J, Sohal RS, Orr WC. (2010) Mitochondrial peroxiredoxins are critical for the maintenance of redox state and the survival of adult Drosophila. Free Radic Biol Med. 49: 1892-1902.
Radyuk, S. N., Michalak, K., Klichko, V. I., Benes, J., Orr, W. C. (2010). Peroxiredoxin 5 modulates immune response in Drosophila. Biochim. et Biophys. Acta 1800: 1153-1163.
Radyuk, S. N., Michalak, K., Klichko, V. I., Benes, J., Rebrin, I., Sohal, R. S. and Orr, W. C. (2009). Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem J. 419(2): 437-445.
Bromberg L., S. Raduyk , T. A. Hatton (2009). Functional Magnetic Nanoparticles for Biodefense and Biological Threat Monitoring and Surveillance, Analytical Chemistry, 81, 5637-5645.
Legan, S. K., Rebrin, I., Mockett, R. J., Radyuk, S. N., Klichko, V. I., Sohal, R. S., Orr, W. C. (2008). Overexpression of Glucose-6-phosphate Dehydrogenase Extends the Life Span of Drosophila melanogaster. J Biol Chem. 283:32492-32499.
Michalak, K., Orr, W. C., Radyuk, S. N. (2008). Drosophila peroxiredoxin 5 is the second gene in a dicistronic operon. Biochem. Biophys. Res. Commun. 368:273-278.
Luchak, J. M., Prabhudesai, L., Sohal, R. S., Radyuk, S. N., and Orr, W. C. (2007) Modulating longevity in Drosophila by over- and underexpression of glutamate-cysteine ligase. Ann. N.Y. Acad. Sci. 1119:260-273.
Radyuk, S. N., Michalak, K., Rebrin, I., Sohal, R. S., Orr, W. C. (2006). Effects of ectopic expression of Drosophila DNA glycosylases dOgg1 and RpS3 in mitochondria. Free Radic Biol Med. 41: 757-764.
Orr, W. C., Radyuk, S. N., Prabhudesai, L., Toroser, D., Benes, J. J., Luchak, J. M., Mockett, R. J., Rebrin, I., Hubbard, J. G. and Sohal, R. S. (2005) Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J. Biol. Chem. 280: 37331-37338.
Radyuk, S. N., Klichko, V. I. and Orr, W. C. (2004) Profiling Cu,Zn-superoxide dismutase expression in Drosophila melanogaster – a critical regulatory role for intron/exon sequence within the coding domain. Gene, 17: 328-48.
Klichko, V. I., Radyuk, S. N. and Orr, W. C. (2004) Profiling catalase gene expression in Drosophila melanogaster during development and aging. Arch. Insect Biochem. Physiol. 56: 34-50.
Radyuk, S. N., Mericko, P. A., Popova, T. G., Grene, E. and Alibek, K. (2003) In vitro-generated respiratory mucosa: a new tool to study inhalational anthrax. Biochem. Biophys. Res. Commun. 305: 624-632.
Radyuk S. N., Sohal R. S. and Orr W. C. (2003) Thioredoxin peroxidases can foster cytoprotection or cell death in response to different stressors - a study of thioredoxin peroxidase under- and over-expression in Drosophila cells. Biochem. J. 371: 743-752.
Mockett R. J., Radyuk S. N., Benes J. J., Orr W. C. and Sohal, R. S. (2003) Phenotypic effects of familial amyotrophic lateral sclerosis mutant Sod alleles in transgenic Drosophila. Proc. Natl. Acad. Sci. U.S.A. 100: 301-306.
Radyuk, S. N., Klichko, V. I., Spinola, B., Sohal, R. S. and Orr, W. C. (2001) The peroxiredoxin gene family in Drosophila melanogaster. Free Radic. Biol. Med. 31: 1090-1100.
Radyuk, S. N., Klichko, V. I. and Orr, W. C. (2000) Catalase expression in Drosophila melanogaster is responsive to ecdysone and exhibits both transcriptional and post-transcriptional regulation. Arch. Insect Biochem. Physiol. 45: 79-93.
Klichko, V. I., Radyuk, S. N. and Orr, W.C. (1999) CuZn-SOD promoter-driven expression in the Drosophila central nervous system. Neurobiol. Aging, 20: 537-543.
Mockett, R. J., Orr, W. C., Rahmandar, J. J., Benes, J.J., Radyuk, S.N., Klichko, V. I. and Sohal, R. S. (1999) Overexpression of Mn-containing superoxide dismutase in transgenic Drosophila melanogaster. Arch. Biochem. Biophys. 371: 260-269.
Candas, M., Sohal, R. S., Radyuk, S. N., Klichko, V. I. and Orr, W. C. (1997) Molecular Organization of the Glutathione Reductase Gene in Drozophila Melanogaster. Arch.Biochem.Bioph. 339: 323-334.